About Our Research
NeuroImaging Lab Research Projects, Selected Publications and Virtual Tour
Research Projects
-
Assessing brain function and dysfunction can be achieved by monitoring blood and nutrient supply as well as clearance of byproducts.
Our lab’s faculty members are deeply invested in this area of research using different technological approaches to cover the entire brain. Dr. Vazquez focuses on cortical areas using tools like optogenetics. Dr. Fukuda focuses on other brain regions like olfactory bulb where there is clear segregation of cellular components. Dr. Poplowsky focuses on MRI-based approaches.
We also work closely with colleagues in this area, especially Dr. Sarah Ross, Dr. Ken Fish and Dr. Jana Kainerstorfer.
More details in the sections below.
-
Our studies on neurovascular regulation have provided detailed insight into the neurophysiology of the healthy brain. We use animal models of disease to study neuro-vascular dysfunction in vascular contributions to cognitive impairment and dementia (VCID) as well as Alzheimer’s Disease (AD).
Early detection is paramount to assess changes in brain function suggestive of advancing neurodegeneration. Vascular dysfunction can play an important role when combined with neurophysiological biomarkers of neuronal dysfunction. We have ongoing projects to determine whether novel treatment strategies can be used to delay AD pathology and serve as a disease management strategy. Dr. Vazquez also examines the impact of reduced perfusion and increased inflammation on the integrity of the neuro-vascalar unit.
We conduct these projects in collaboration with our local ADRC and our colleagues in Bioengineering, Neurobiology, Neurology and Psychiatry; especially Dr. Bistra Iordanova, Dr. Milos Ikonomovic, Dr. William Klunk, Dr. Oscar Lopez, and Dr. Xiaoming Hu.
We also work with Dr. Manole of Pediatrics to characterize the impact of cardiac arrest on brain health.
-
We work closely with Dr. Cui and Dr. Kozai of Bioengineering to study the long-term impact of neural devices to readout and modulate brain activity. The Cui Lab continuously innovates materials that promote device integration, sensing, drug delivery and stimulation, while the Kozai Lab has tremendous expertise characterizing the interplay and impact of neural devices on brain function.
Recent Research Images
Neurovascular Regulation
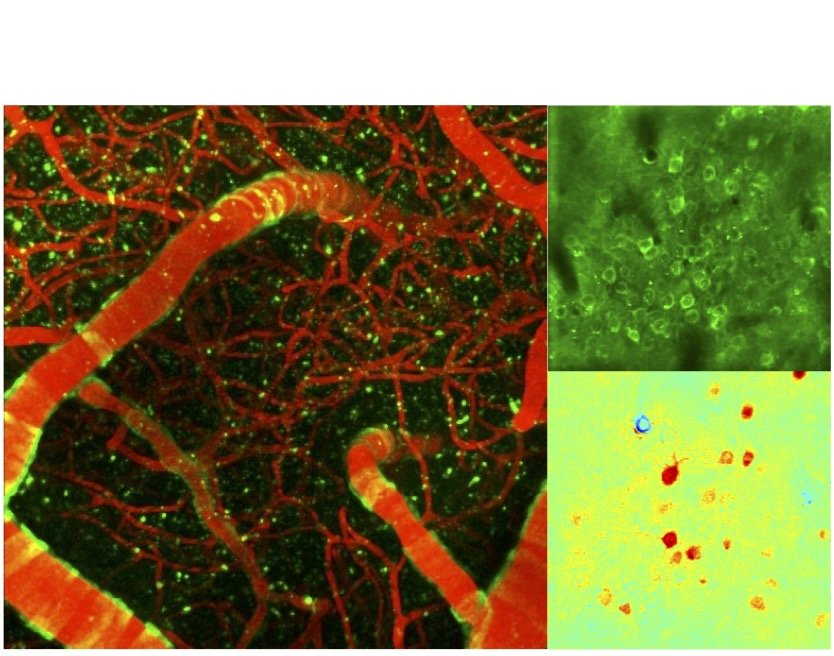
Transgenic mice make possible simultaneous imaging of neuronal activity through different fluorescent sensors concurrently with vascular and metabolic imaging through intrinsic imaging. We conduct these experiments routinely over one or both brain hemispheres.
Neuronal, vascular and metabolic imaging
Our optical readouts are carefully selected to match sensitivities that can also be obtained using Magnetic Resonance Imaging (MRI). Our group has conducted extensive cell-type specific studies by MRI in the olfactory bulb using BOLD- and CBV-weighted imaging. MRI provides a whole-brain perspective into our studies.
Functional Magnetic Resonance Imaging
Local Neurovascular Interactions
This video shows high-resolution images of the mouse cortex acquired using two-photon imaging in awake mice. Activity of neurons is evident and arterial diameter changes are also observed. We want to understand everything underlying this relationship.
Optogenetics
We use this tool and high-resolution imaging (two-photon) to evaluate the vaso-regulatory capacity of different neurons. In this video, we have targeted NOS-expressing inhibitory neurons in barrel cortex of NG2 mice and optogenetically activated them while imaging nearby vasculature (red color). As you can see near the end of the video, when the optogenetic stimulus is presented (yellow bar), arteries dilate, indicating that these neurons are strong regulators of local vasculature.
Neurodegeneration
Progressive neurodegenerative diseases like Alzheimer’s Disease and Related Dementias (ADRD) take years if not decades before irreversible changes, like neuronal death, take place. In the earlier stages of these diseases, neuronal processing is altered, offering an opportunity for early detection. We identify physiologically relevant markers of neuronal and vascular dysfunction that when combined with routine clinical exams (for example, FDG- and amyloid-PET, MRI/fMRI) can provide a clearer picture of potential disease stage. We combine our optical methods with clinical tools for this project.
Early Detection
Neuroengineering
Enhancing Brain Perfusion
We are developing pharmacological and device-based approaches to enhance or promote brain health by manipulating brain vasculature. Understanding the role and propagation of neurovascular rhythms is a fundamental aspect of this project. We use optogenetics and chemogenetics as test platforms to evaluate the potential efficacy of the approaches.
Neural Devices and Brain Function
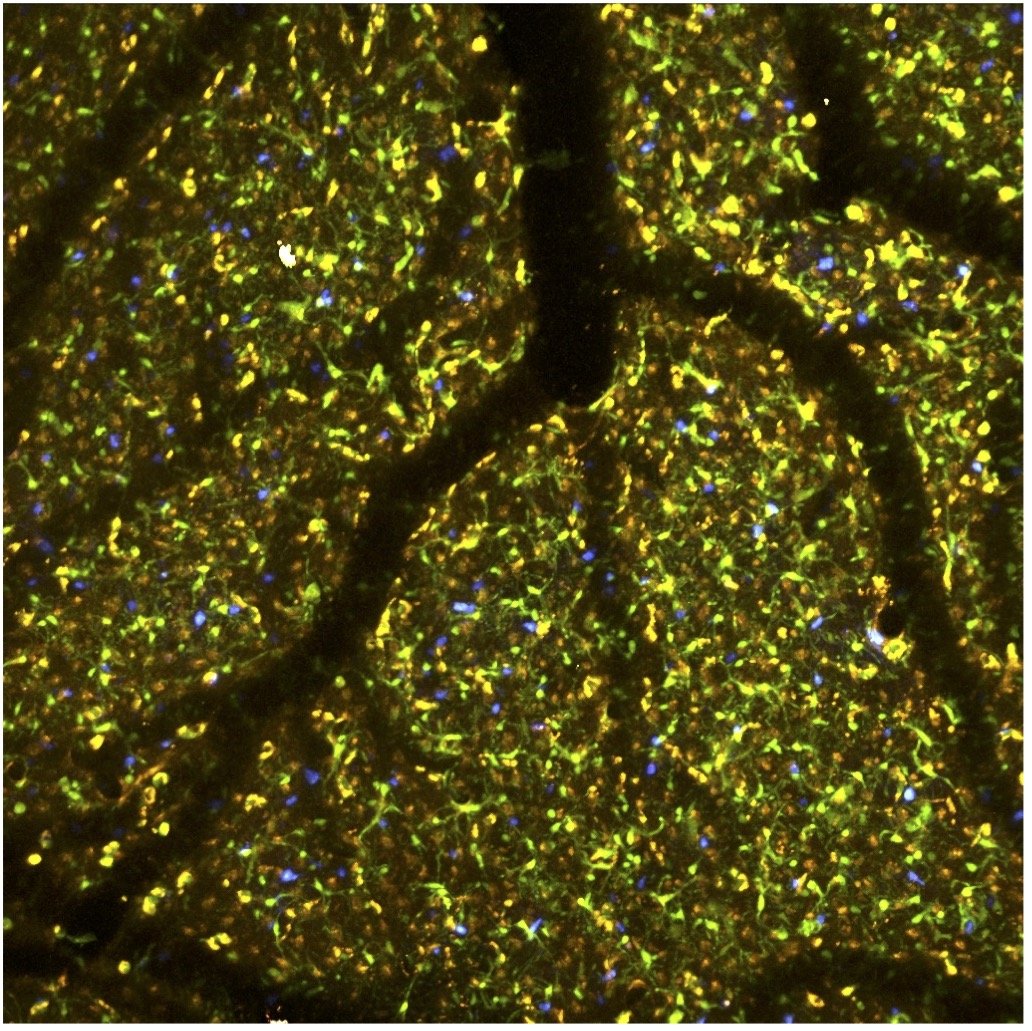
We work closely with Dr. Tracy Cui and Dr. Takashi Kozai of Bioengineering understanding the impact of neural devices on brain function. We use transgenic mouse models and two-photon imaging to visualize the impact of these devices on neurons, vascular anatomy and function, BBB integrity and immune responses. We also study the impact and efficacy of micro-stimulation devices on network activity. In a project with Dr. Kozai we also examine how small loose devices can be used to stimulate brain tissue via photo-conversion.
Other Collaborations
Cardiac arrest is not only a problem of the heart, it also has an adverse impact on other organs, especially those that require high levels of blood perfusion like the brain. We are characterizing the impact of cardiac arrest on brain physiology, including neuronal and vascular health, upregulation of immune response, and BBB disruption. We are using this knowledge to develop clinically relevant strategies to improve neurological outcome.
Recovery after Cardiac Arrest.
Selected Publications
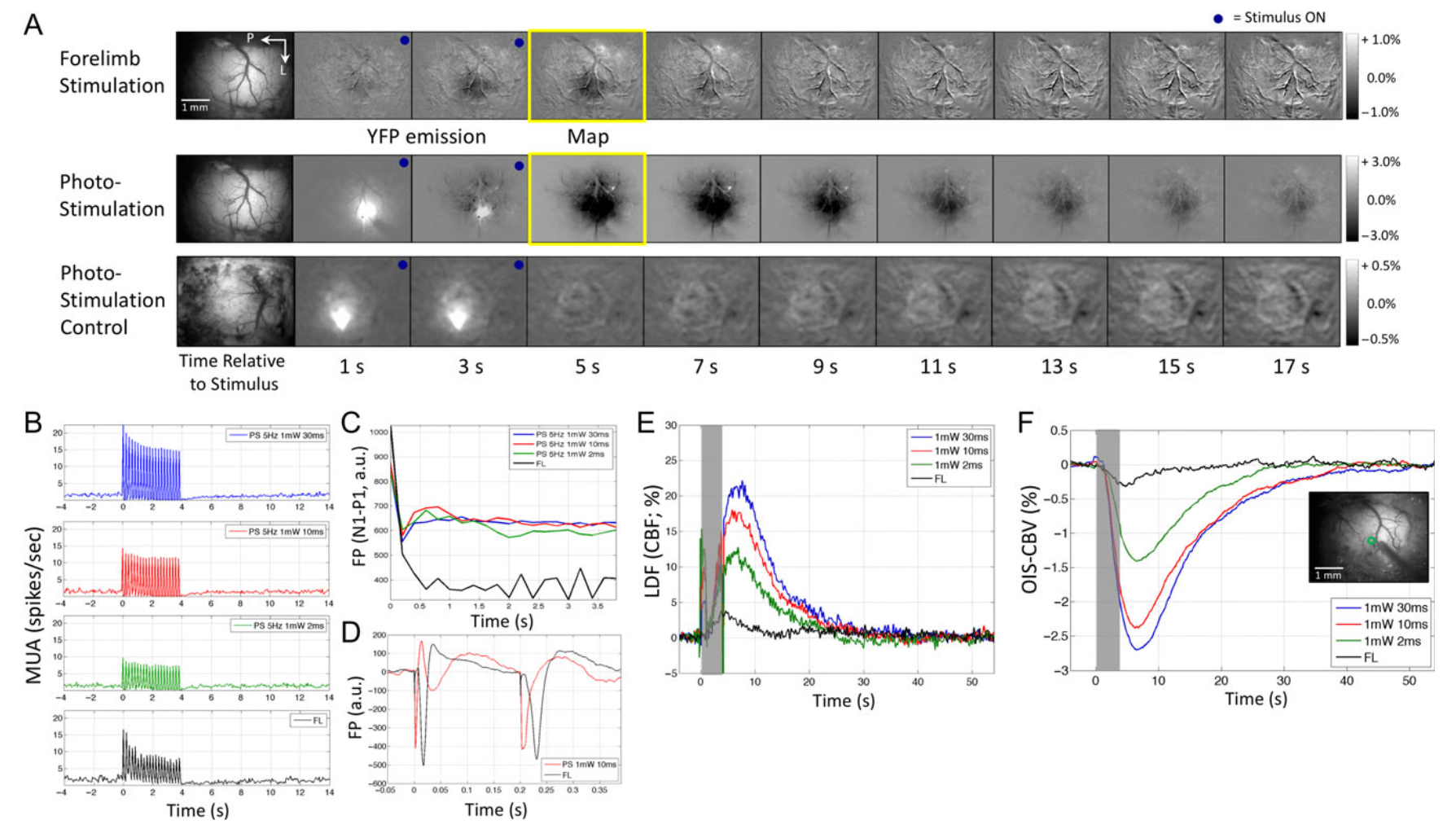
Neural and hemodynamic responses elicited by forelimb and photo-stimulation
-
Vazquez AL, Fukuda M, Crowley JC, Kim SG. Neural and hemodynamic responses elicited by forelimb- and photo-stimulation in channelrhodopsin-2 mice: insights into the hemodynamic point spread function. Cereb Cortex. 2014 Nov;24(11):2908-19. doi: 10.1093/cercor/bht147. Epub 2013 Jun 12. PMID: 23761666; PMCID: PMC4193461.
-
Hemodynamic responses are commonly used to map brain activity; however, their spatial limits have remained unclear because of the lack of a well-defined and malleable spatial stimulus. To examine the properties of neural activity and hemodynamic responses, multiunit activity, local field potential, cerebral blood volume (CBV)-sensitive optical imaging, and laser Doppler flowmetry were measured from the somatosensory cortex of transgenic mice expressing Channelrhodopsin-2 in cortex Layer 5 pyramidal neurons. The magnitude and extent of neural and hemodynamic responses were modulated using different photo-stimulation parameters and compared with those induced by somatosensory stimulation. Photo-stimulation-evoked spiking activity across cortical layers was similar to forelimb stimulation, although their activity originated in different layers. Hemodynamic responses induced by forelimb- and photo-stimulation were similar in magnitude and shape, although the former were slightly larger in amplitude and wider in extent. Altogether, the neurovascular relationship differed between these 2 stimulation pathways, but photo-stimulation-evoked changes in neural and hemodynamic activities were linearly correlated. Hemodynamic point spread functions were estimated from the photo-stimulation data and its full-width at half-maximum ranged between 103 and 175 µm. Therefore, submillimeter functional structures separated by a few hundred micrometers may be resolved using hemodynamic methods, such as optical imaging and functional magnetic resonance imaging.
-
Dominance of layer-specific microvessel dilation in high-resolution fMRI
-
Poplawsky AJ, Fukuda M, Kang BM, Kim JH, Suh M, Kim SG. Dominance of layer-specific microvessel dilation in contrast-enhanced high-resolution fMRI: Comparison between hemodynamic spread and vascular architecture with CLARITY. Neuroimage. 2019 Aug 15;197:657-667. doi: 10.1016/j.neuroimage.2017.08.046. Epub 2017 Aug 16. PMID: 28822749; PMCID: PMC5815958
-
Contrast-enhanced cerebral blood volume-weighted (CBVw) fMRI response peaks are specific to the layer of evoked synaptic activity (Poplawsky et al., 2015), but the spatial resolution limit of CBVw fMRI is unknown. In this study, we measured the laminar spread of the CBVw fMRI evoked response in the external plexiform layer (EPL, 265 ± 65 μm anatomical thickness, mean ± SD, n = 30 locations from 5 rats) of the rat olfactory bulb during electrical stimulation of the lateral olfactory tract and examined its potential vascular source. First, we obtained the evoked CBVw fMRI responses with a 55 × 55 μm2 in-plane resolution and a 500-μm thickness at 9.4 T, and found that the fMRI signal peaked predominantly in the inner half of EPL (136 ± 54 μm anatomical thickness). The mean full-width at half-maximum of these fMRI peaks was 347 ± 102 μm and the functional spread was approximately 100 or 200 μm when the effects of the laminar thicknesses of EPL or inner EPL were removed, respectively. Second, we visualized the vascular architecture of EPL from a different rat using a Clear Lipid-exchanged Anatomically Rigid Imaging/immunostaining-compatible Tissue hYdrogel (CLARITY)-based tissue preparation method and confocal microscopy. Microvascular segments with an outer diameter of <11 μm accounted for 64.3% of the total vascular volume within EPL and had a mean segment length of 55 ± 40 μm (n = 472). Additionally, vessels that crossed the EPL border had a mean segment length outside of EPL equal to 73 ± 61 μm (n = 28), which is comparable to half of the functional spread (50-100 μm). Therefore, we conclude that dilation of these microvessels, including capillaries, likely dominate the CBVw fMRI response and that the biological limit of the fMRI spatial resolution is approximately the average length of 1-2 microvessel segments, which may be sufficient for examining sublaminar circuits.
-
Development of a PET radioligand selective for cerebral amyloid angiopathy
-
Abrahamson EE, Stehouwer JS, Vazquez AL, Huang GF, Mason NS, Lopresti BJ, Klunk WE, Mathis CA, Ikonomovic MD. Development of a PET radioligand selective for cerebral amyloid angiopathy. Nucl Med Biol. 2021 Jan;92:85-96. doi: 10.1016/j.nucmedbio.2020.05.001. Epub 2020 May 12. PMID: 32471773
-
Positron emission tomography (PET) using radiolabeled amyloid-binding compounds has advanced the field of Alzheimer's disease (AD) by enabling detection and longitudinal tracking of fibrillar amyloid-β (Aβ) deposits in living people. However, this technique cannot distinguish between Aβ deposits in brain parenchyma (amyloid plaques) from those in blood vessels (cerebral amyloid angiopathy, CAA). Development of a PET radioligand capable of selectively detecting CAA would help clarify its contribution to global brain amyloidosis and clinical symptoms in AD and would help to characterize side-effects of anti-Aβ immunotherapies in AD patients, such as CAA. A candidate CAA-selective compound (1) from a panel of analogues of the amyloid-binding dye Congo red was synthesized. The binding affinity to Aβ fibrils and lipophilicity of compound 1 were determined and selectivity for CAA versus parenchymal plaque deposits was assessed ex-vivo and in-vivo in transgenic APP/PS1 mice and in postmortem human brain affected with AD pathology. Compound 1 displays characteristics of Aβ binding dyes, such as thioflavin-S, in that it labels both parenchymal Aβ plaques and CAA when applied to histological sections from both a transgenic APP/PS1 mouse model of Aβ amyloidosis and AD brain. Thus, compound 1 lacks molecular selectivity to distinguish Aβ deposits in CAA from those in plaques. However, when administered to living APP/PS1 mice intravenously, compound 1 preferentially labels CAA when assessed using in-vivo two-photon microscopy and ex-vivo histology and autoradiography. We hypothesize that selectivity of compound 1 for CAA is attributable to its limited penetration of the blood-brain barrier due to the highly polar nature of the carboxylate moiety, thereby limiting access to parenchymal plaques and promoting selective in-vivo labeling of Aβ deposits in the vascular wall.
-
Alberto’s Lab Tour
General tour of the lab. Alberto gives some background on what motivates our work. There is also a brief description of our projects, facilities and personnel.
Adiya’s Lab Tour
One of our graduate students does fantastic job at touring the lab and our facilities as if you were here visiting.